Dr Darren Hudson tells us about his recent project, using Patient and Public Involvement and Engagement (PPIE) Participant Payment Funding to inform studies into the effectiveness of a new MRI contrast agent. Working alongside people with lived experience, the project used public engagement to inform the future shape of this research.
Amount of award: £300
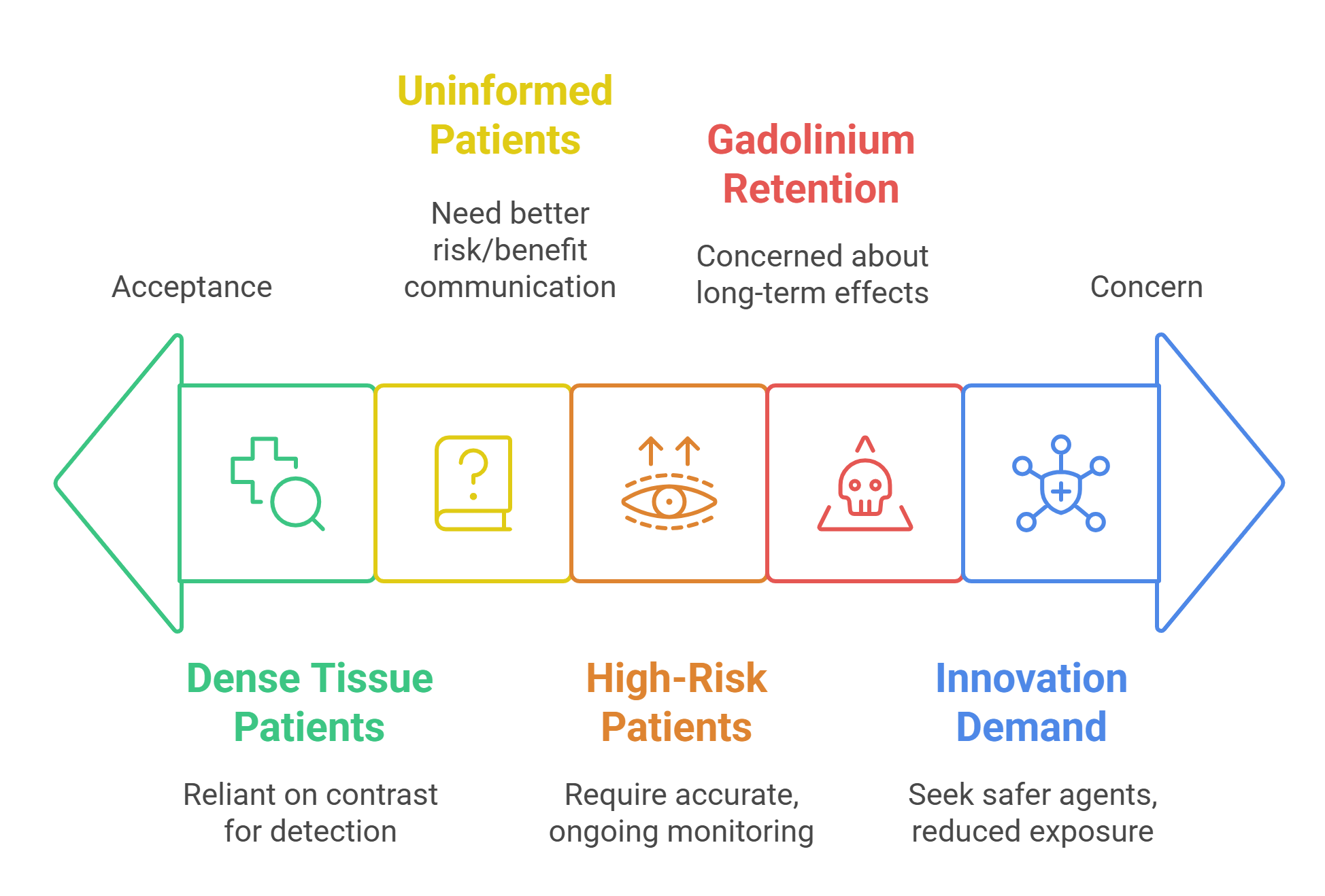
Our research aims to evaluate the clinical effectiveness of Elucirem, a new gadolinium-based contrast agent, specifically within breast MRI imaging. Gadolinium-based contrast agents can help provide greater clarity in an MRI, and are particularly useful for detecting focal lesions.
Including patients and the public from the outset was essential to ensure the research reflects the experiences, concerns, and priorities of those directly impacted. The motivation behind PPIE was not only to enhance scientific rigour but also to co-develop a study grounded in real-world relevance, accessibility, and trustworthiness: factors that only those with lived experience can truly provide.
With support from the University of Exeter’s internal Patient and Public Involvement and Engagement (PPIE) Participant Payment Fund, we hosted an initial online engagement event via Microsoft Teams. Attendees included individuals with lived experience of breast cancer and breast MRI recruited through Breast Cancer Now, alongside representatives from Guerbet UK. The session introduced the concept of Elucirem and invited attendees to share their thoughts on contrast safety, study design acceptability, and informational needs. Contributors also had the opportunity to provide further input via Padlet, helping us capture a breadth of perspectives.
One key challenge was ensuring accessibility, particularly balancing the technical nature of MRI and contrast use with clear, understandable communication. Feedback highlighted the importance of simplifying language and using visual aids. The session gave us a new understanding of what aspects were important to patients and what approaches were seen as most acceptable.
Personally, I found gaining this deeper appreciation of what matters most to patients was the greatest benefit of this project. For the research itself, the engagement activity has helped shape more feasible study designs and revealed the importance of co-developing educational materials. For participants, it offered a rare chance to influence future imaging practice and raise awareness of issues often overlooked in clinical care, such as communication about contrast agents.
We plan to integrate this patient feedback into the next stages of study development, including co-designing patient information materials and refining our research methodology. The engagement has laid a strong foundation for continued involvement throughout the project lifecycle. Longer term, we hope this collaborative approach will improve not only the implementation of safer contrast agents but also the way information is shared with patients, empowering them to be better informed and more confident in their care.