Team Smirnoff
Nick Smirnoff is interested in the functions of reactive oxygen species (ROS, such as hydrogen peroxide) and antioxidants in plants. ROS, produced by electron transport chains and oxidase enzymes, are used as signalling molecules. He is using an organelle-targeted genetically encoded hydrogen peroxide sensor (roGFP2-Orp1) in Arabidopsis thaliana to investigate its production and function during high light acclimation, pathogen infection and pollen-stigma self-incompatibility.
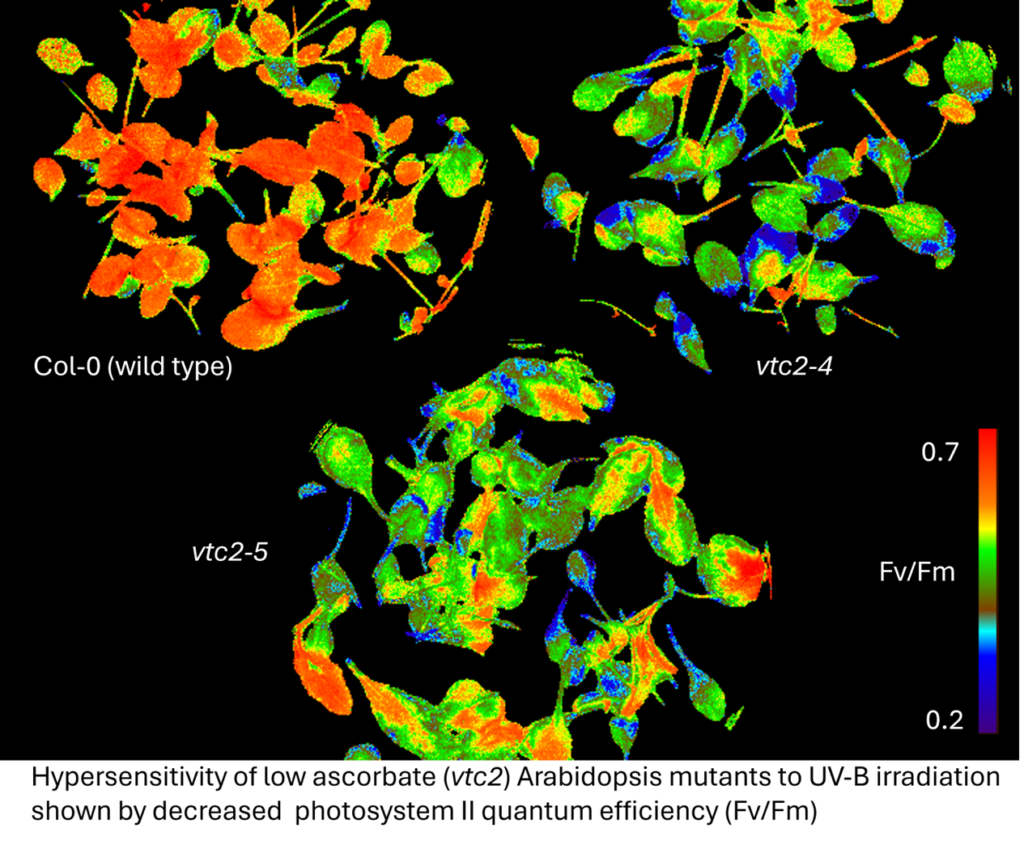
Ascorbate (vitamin C) is a multifunctional antioxidant and a chaperone for 2-oxoglutarate-dependent dioxygenase enzymes. We have a long-term interest in understanding ascorbate metabolism and functions in plants and algae. Current work is focussed on GDP-L-galactose phosphorylase, the rate-controlling biosynthetic enzyme, particularly translational control of its expression by an upstream open reading frame (uORF) and transcriptional control by light in Arabidopsis thaliana and tomato. Fungi make an ascorbate analogue, erythroascorbate, and we are investigating its functions in plant pathogenic fungi.
Ergothioneine and ovothiols are thiol/thione derivatives of histidine with proposed antioxidant/cytoprotective roles. Ergothioneine is made by fungi and some bacteria, but we have found it is produced by green/red algae, bryophytes and ferns and we are using the model liverwort Marchantia polymorpha to understand its role in plants. We are using ergothioneine biosynthesis mutants of plant pathogenic fungi to investigate proposed roles in pathogenicity. Ovothiols are produced by diatoms and other marine organisms and are being investigated in Phaeodactylum tricornutum.
Team Kumar

Our research is addressing the fundamental biological question of how plants sense and integrate environmental signals. We are particularly interested in understanding the thermosensory modulation of growth and immunity. Current research is seeking to understand the genetic and epigenetic mechanisms of gene regulation in response to the environment; and adaptation of these mechanisms to local and dynamic environments. Our long-term objective is to provide a mechanistic framework for temperature perception and its impact on growth and defense responses. We are interested in elucidating the fundamental principles that underlie important biological processes such as environmental signal integration and trade-offs between important traits. Beyond fundamental biology, our research will contribute to the efforts to improve yield, particularly through laying foundation for climate resilient crops in the long run.
Team Johnson
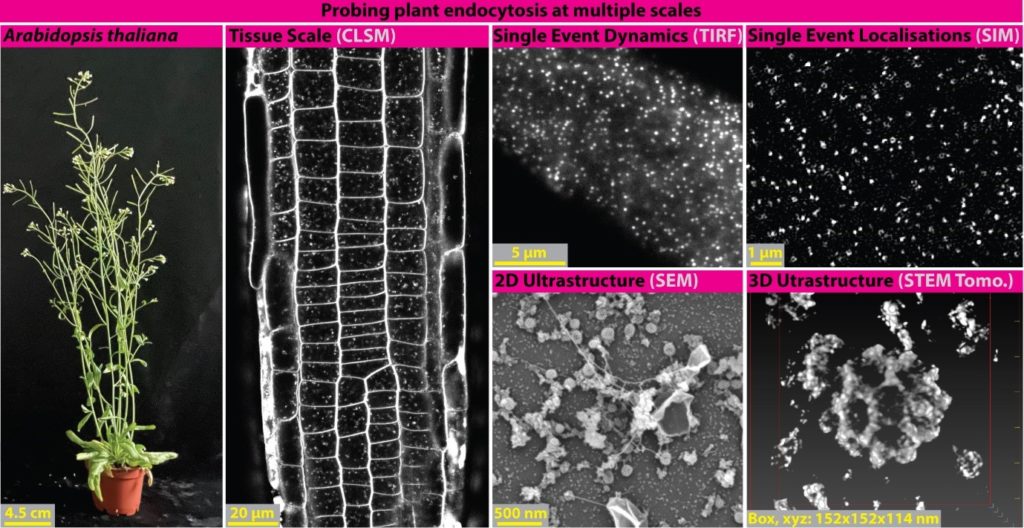
Alex Johnson researches the fundamental eukaryotic cell biology processes regulating cellular sensitivity to environmental stimuli, specifically endocytic mechanisms and their roles in receptor internalisation in plants. Plants provide our staple food, nutrition, and oxygen, so understanding how they mediate sensitivity to the environment is essential for maintaining human wellbeing. As sessile organisms, they are exposed to changing conditions and must modulate their sensitivity in response. Endocytosis pathways, such as Clathrin-mediated endocytosis CME), are central to this process. Despite CME’s physiological significance, it remains poorly understood in plants. However, recent work has found that it is evolutionarily and mechanistically distinct from other model systems, highlighting critical gaps in our understanding of eukaryotic CME. Using a range of quantitative microscopy approaches, we examine plant endocytosis at tissue (confocal), live single-event (TIRF and super-resolution SIM), and ultrastructural (SEM and STEM tomography) scales. By combining these tools with biochemistry (interactomes, proteomes, in vitro reconstitution assays) and biophysical imaging (Brillouin light scattering), we are investigating: 1 – How single events of CME work? 2 – How CME mediates specific environmental responses? 3 – How plant endocytosis compares to other eukaryotic models? Together, these will provide a new understanding of how plants sense and adapt to their environment, at the molecular level and beyond.
Team Bauer – The Exeter Mechanical Ecology Lab

We use an interdisciplinary approach to study how plants deal with the physics in their live. We want to understand how plants deal with physical challenges such as wind, rain and hail, and how some plants take advantage of physical principles to capture or repel insects or direct water across leaf surfaces. We work a lot with carnivorous pitcher plants because they are an excellent model to study the function, development and evolution of plant surface adaptations and their ecological implications. We use a very broad range of experimental, analytical and theoretical approaches including field and lab experiments, biomechanics, bioimaging, molecular biology and transcriptomics, analytical chemistry and finite element modelling.
Team Nützmann
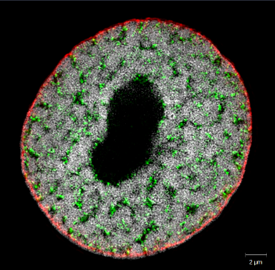
Our lab investigates genome organisation, epigenetic gene regulation and chromosome architecture in the context of plant and microbial metabolism, immunity and evolution. Find more information at https://genorglab.wordpress.com/.
Team Fones
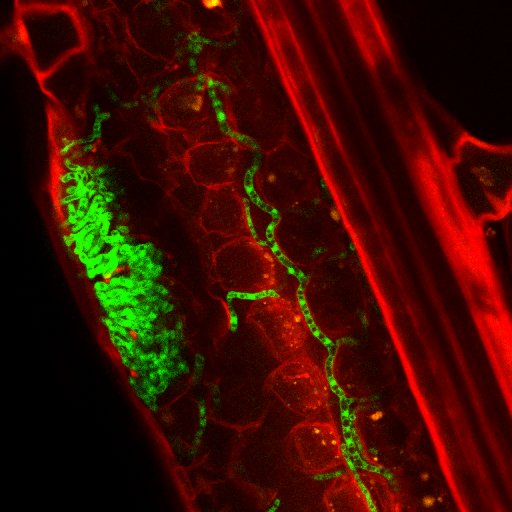
Research the Fones lab focusses on the fungus Zymoseptoria tritici – the causal agent of Septoria tritici blotch (STB) disease in wheat. We discovered that Zymoseptoria spends its first ~10 days of infection growing over the leaf surface. Through microscopic & molecular characterisation of >50 reference & field isolates of Zymoseptoria on wheat cultivars of differing levels of resistance, we aim to understand fungal leaf surface growth: why it happens, how the fungus obtains nutrients from the plant to fuel it, and how surface fungus interacts with the plant immune system. We hope to uncover fungal vulnerabilities and develop novel methods to control STB disease. The need for this is pressing, as fungicide resistance is rapidly emerging in Zymoseptoria.
We also study the leaf surface microbiome of field-grown wheat with a view to finding out how other microbes influence disease. We have discovered that Zymoseptoria is capable of growing as a biofilm, alone or with microbial partners, and we are currently using Exeter’s state-of-the art “Global meteorological simulator” (GMS) to determine what weather conditions promote leaf-surface biofilms and how this affects fungal survival during stressful conditions like hot weather, drying, or fungicide application. Using combinations of electron and confocal microscopy along with metabolomic analyses and stimulated Raman spectroscopy (SRS) we are investigating the properties of Zymoseptoria biofilms, how and from what they are formed, and how molecules secreted by partner microbes affect fungal interactions with the wheat leaf.
More about us and our research: https://sites.exeter.ac.uk/plantsatexeter/the-fones-lab/
Team Deeks
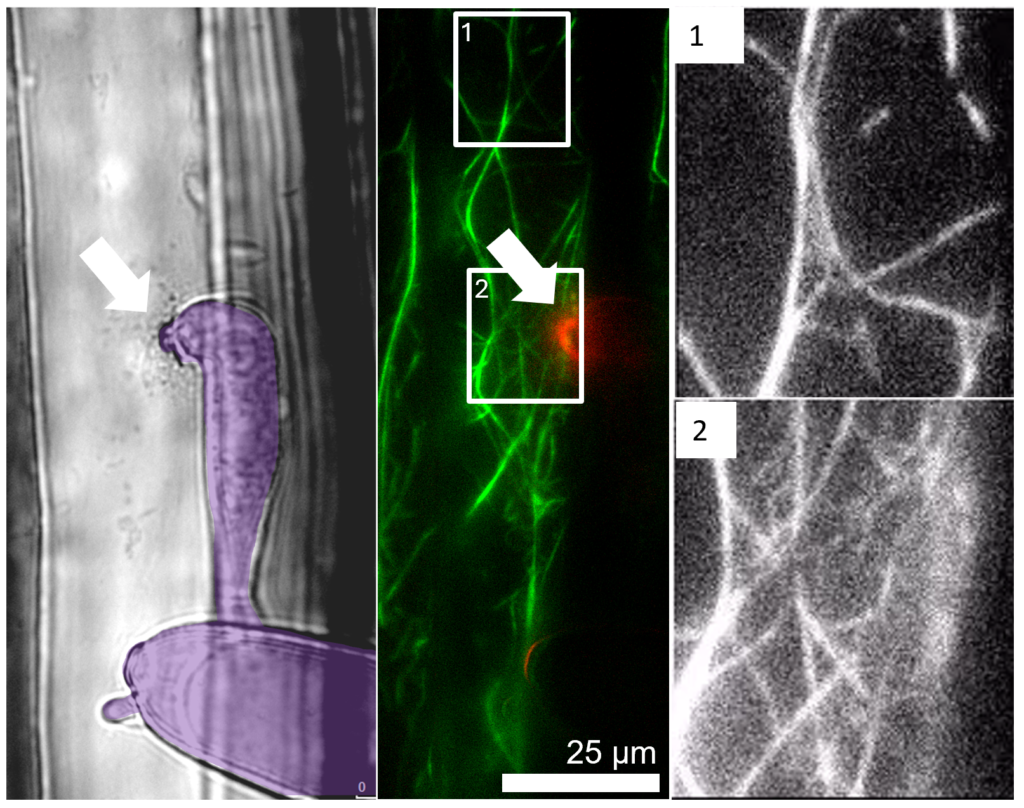
We are interested in the way plant cells respond to spatial information to guide immune responses. The cells of plants have an acute awareness of the events occurring immediately beyond their walls and make spatially precise responses to any perceived threat. Potentially pathogenic microbes trigger plant defences to focus within a few square microns of the plant cell surface. This process can be summarised as ‘pathogen-oriented cell polarisation’. The response is effective against most microbes but successful pathogens evade these counter-measures and establish infection. Our team aims to understand how multiple cell processes (the cytoskeleton, trafficking and receptor signalling) are co-ordinated as a system to achieve this important response that underpins the plant immune system. For instance we have discovered that cargo delivery and actin networks are closely coupled through FORMIN proteins during this process. This research is an interdisciplinary challenge involving multiple collaborators in different fields.

We also work closely with the Fones and Smirnoff teams to understand how Zymoseptoria tritici and other fungal pathogens survive under the stresses imposed by the plant immune system. Furthermore we are collaborating with the physicist Wolfram Moebius to investigate at multiple scales how viruses migrate through a plant and how their genetics are affected by the physical constraints they encounter during this complex journey. The video to the left shows whole-plant fluorescence imaging of an infection using three strains of Tobacco Etch Virus (TEV) encoding different fluorescent colours (red, green and blue). More about us and our research: https://sites.exeter.ac.uk/plantsatexeter/the-deeks-lab/
Team Gudelj
Ivana Gudelj’s group uses mathematical and experimental approaches to study competition, cooperation, and coevolution among microorganisms. They are a truly interdisciplinary team where mathematicians, physicists, bioinformaticians, molecular biologists, and experimental evolutionary ecologists, work together and share expertise. This collaborative environment is ideal for training the next generation of scientists, equipping them to tackle complex cross-disciplinary challenges in evolutionary ecology.
By integrating systems biology with population-level frameworks, the group aims to quantify how microbial community composition is shaped by the metabolism, genetics, and physiology of individual organisms, and how these factors interact with and how these factors interact with abiotic conditions such as temperature, humidity, wind, and rainfall. This work addresses fundamental eco-evolutionary problems, including the evolution of diversity, disease transmission and virulence, and the emergence of antimicrobial resistance.
Research highlights
Plant Disease Transmission under Climate Stresses
Climate change is reshaping when and where fungal pathogens thrive, increasing the diversity of threats to crops and putting global food security at risk. To better predict and manage these risks, we study rice and its fungal pathogen Magnaporthe oryzae using mathematical models and transmission experiments in our new Global Meteorological Simulator (GMS).
Effects of Multi-Trait Interactions on Pathogen Community Function in Plants
Microbial traits rarely act in isolation; their interactions can produce surprising outcomes that complicate plant disease management. By studying synthetic microbial communities—including the rice pathogen Magnaporthe oryzae—and combining them with multi-trait mathematical models, we uncover how these interactions shape pathogen growth, transmission, and virulence.
Phage Therapy as a Biocontrol Strategy for Plant Pathogens
Bacteria–phage interactions help shape how bacterial plant diseases spread and evolve. Using rice, its bacterial pathogen Xanthomonas oryzae, and the phage Bosa, we combine experimental evolution with modelling to uncover how coevolution and transmission dynamics influence disease outcomes and potential biocontrol strategies.