Haemochromatosis: genetic iron overload disease
Summary for patients
Haemochromatosis: genetic iron overload disease
Summary for patients
You have one copy of the HFE C282Y genetic variant.
* Estimates are from our community sample of UK Biobank European descent individuals [1]. People tested because of a health problem or with high iron levels may have different risk. See below Technical Details section for more information, and the Other Risk Factors page for risk modifiers. Page updated 25th May 2023.
Males are known to have higher risk of iron overload disease compared to females.
Haemochromatosis – 0.22% of men with one copy of the HFE C282Y gene variant were diagnosed with haemochromatosis. In comparison, 0.06% of men without a faulty HFE gene were diagnosed. Data from the UK Biobank participants linked medical records up to Jan 2018 [1].
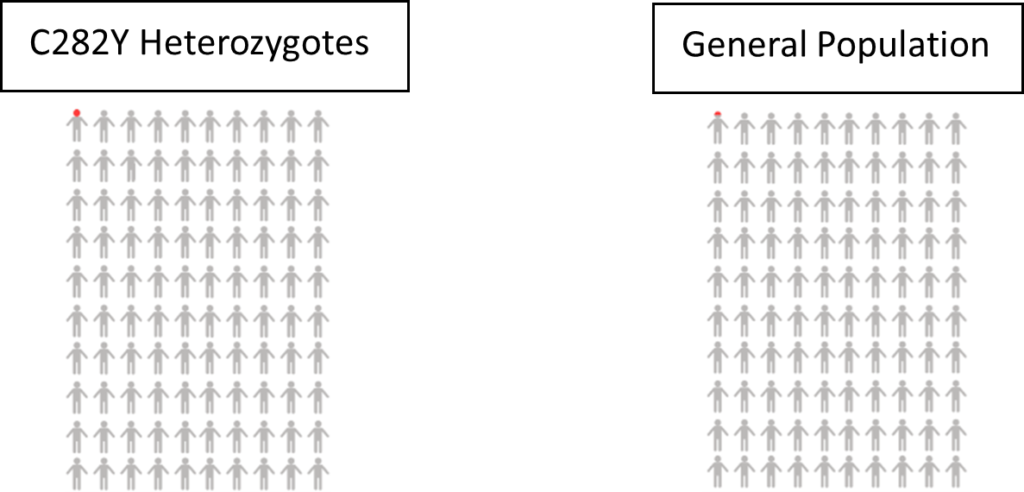
Risk of disease/mortality – In our study of mortality and disease risk to age 75 in UK Biobank, males with one copy of the HFE C282Y gene variant did not have increased risk of death or liver complications (including liver disease and liver cancer).
Haemochromatosis – 0.08% of women with one copy of the HFE C282Y gene variant were diagnosed with iron overload-related haemochromatosis. In comparison, only 0.02% of women without a faulty HFE gene were diagnosed.
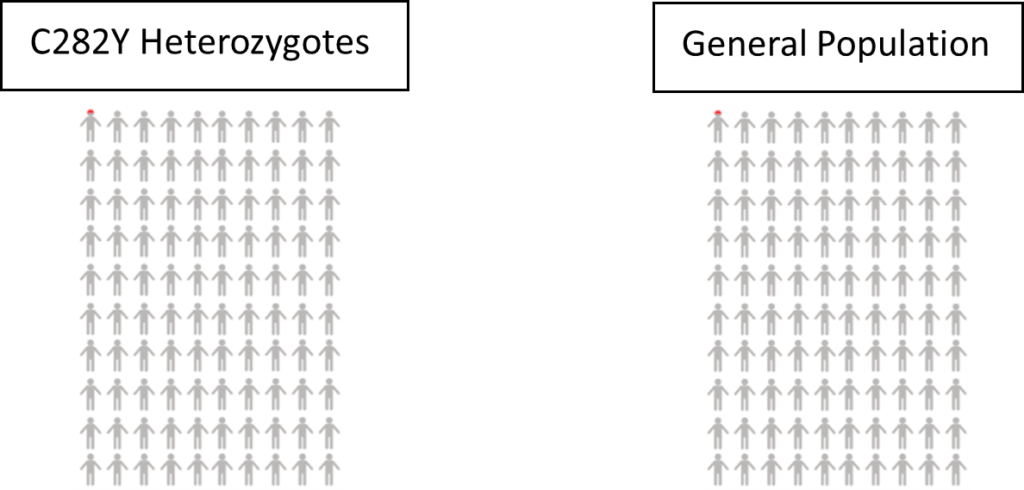
Risk of disease/mortality – In our study of mortality and disease risk to age 75 in UK Biobank, females with one copy of the HFE C282Y gene variant did not have increased risk of death or liver complications (including liver disease and liver cancer).
Because you have one copy of the HFE C282Y gene variant, it is not likely you will develop haemochromatosis; therefore, if you experience symptoms, it is unlikely related to haemochromatosis, but you should contact your GP if they persist or become more severe. See below for guidance:
The UK NHS website for haemochromatosis (link) says to speak to your GP about getting a test if:
1. See below effect estimates for disease and mortality for male and female C282Y heterozygotes, note that all results are statistically non-significant:
2. Genetic differences among different populations can influence the risk of disease prevalence and rates of mortality. Therefore, the generalisability of our study findings may be limited to individuals of European descent.
3. Early follow-up years are liable to be impacted by healthy volunteer bias in the UK Biobank. However, the present study’s findings reported incident outcomes with sufficient follow-up time (median of 8.9 years), thus, limiting the influence of a healthy volunteer bias.
4. The current study does not take into account other genotypes that are known to influence iron metabolism. Therefore, the potential implication of such modifiable effects on iron could have affected any associated risks of disease or mortality. However, further research is required to determine the extent of such effects.
[1] Atkins et al. (2021) “Association of Hemochromatosis HFE p.C282Y Homozygosity With Hepatic Malignancy” JAMA. doi:10.1001/jama.2020.21566